1. a) Phosphoric acid
c) found in Coke Zero
2. a) Calcium Carbonate
c) found in Nutrigrain Bar (strawberry)
3. a) Zinc Oxide
4. a) Potassium Chloride
c) found in Progresso chicken and barley soup
5. a) Sodium Phosphate
c) found in Progresso chicken and barley soup
6. a) Magnesium Sulfate
c) found in One a Day women's vitamin drink mix
7. Manganese Sulfate (II and III)
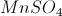 (II)
(II) c) found in One a Day women's vitamin drink mix
8. a) Chromium Chloride
c) found in One a Day women's vitamin drink mix
9. Ammonium Sulfate
c) found in Old London bread crumbs
10. Calcium Sulfate
c) found in Old London bread crumbs
For 10 other items check out Puja's Blog!!!


















